
Related Topics:
More Chemistry Lessons
Stoichiometry Lessons
5.00 L of a gas is known to contain 0.965 mol. If the amount of gas is increased to 1.80 mol, what. Avogadro’s law, which is also called Avogadro’s principle or Avogadro’s hypothesis, only approximates the behavior of real gases because it applies only to an ideal gas. Real gases behave like an ideal gas only under conditions of low temperatures and pressures. The molar volume is close to 22.4 liters (22.4 dm 3) for virtually all gases. That equal volumes of gases at the same temperature and pressure contain equal numbers of molecules was first suggested in 1811 by the Italian chemist Amadeo Avogadro (1776 to 1856). Consequently it is called Avogadro’s law or Avogadro’s hypothesis. The ideal gas law is the equation you must memorize for gases. It not only allows you to relate P, V, n and T, but can replace any of the three classical gas laws in a pinch. For example, let's say you're given constant values of P and n, but forget how Charles' law relates V and T.
In these lessons, we will learn the Molar Volume, Avogadro's Law, how to calculate gas volumes given moles and grams, how to calculate moles given gas volumes and how to calculate gas volumes given the chemical equation.
The molar volume is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure.
There are two standards, commonly used in schools:
- STP (standard temperature and pressure) which is 0° C and 1 atmosphere.
- RTP (room temperature and pressure) which is 25° C and 1 atmosphere.
Molar Volume
Avogadro’s Law states that:
1 mole of every gas occupies the same volume, at the same temperature and pressure.
At STP (standard temperature and pressure), this volume is 22.4 liters
At RTP (room temperature and pressure), this volume is 24 dm3 (liters)
We can also say:
The molar volume of a gas is 22.4 liters at STP (standard temperature and pressure).
The molar volume of gas is 24 dm3 at RTP (room temperature and pressure).
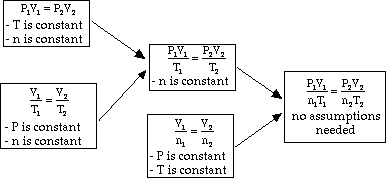
The following diagrams show how to convert between Mass, Moles and Gas Volumes. Scroll down the page for more examples and solutions.How to find the molar volume of a gas using the ideal gas law?
The most common molar volume is the molar volume of an ideal gas at standard temperature and pressure (273 K and 1.00 atm).
The molar volume is the volume occupied by 1 mol of a gas at standard temperature and pressure (STP). It can be calculated using PV = nRT.
Gas volumes from moles and grams
Example:
Calculate the volume of carbon dioxide gas, CO2, occupied by (a) 5 moles and (b) 0.5 moles of the gas occupied at STP.
Solution:
a) Volume of CO2= number of moles of CO2 × 22.4 L
= 5 × 22.4
= 112 L
b) Volume of CO2
= number of moles of CO2 × 22.4 L
= 0.5 × 22.4
= 11.2 L
How to convert from grams to moles to liters?
The following video shows an example of grams to moles to liters conversion.
It shows how to convert grams of a substance to liters at STP.
Example:
What is the volume of 5.643g of CO
Avogadro's Gas Law Examples
s at STP?Moles from Gas Volume
Example:
Calculate the number of moles of ammonia gas, NH3, in a volume of 80 L of the gas measured at STP.
Solution:
Volume of gas = number of moles × 22.414 L/mol
How to convert from liters to moles?
The following video shows an example of liters to moles conversion. It shows how to convert litres of a gas at STP into moles
Example:
How many moles are there in 60.2L of COs at STP?
- Show Step-by-step Solutions
Gas volumes from equations
From the equation for a reaction, we can tell how many moles of a gas take part. Using Avogadro’s Law, we can also work out its volume.
Example:
What volume of hydrogen will react with 22.4 liters of oxygen to form water? (All volumes are measured at STP)
Solution:
Step 1: Write a balanced equation for the reaction.2H2 (g) + O2 (g) → 2H2O (l)
Step 2: Calculate the volume.
From the equation, 2 volumes of hydrogen react with 1 of oxygen or
2 × 22.4 liters of hydrogen react with 22.4 liters of oxygen.
The volume of hydrogen that will react is 44.8 liters.
Example:
When sulfur burns in air it forms sulfur dioxide. What volume of this gas is produced when 1 g of sulfur burns? (Ar : S = 32) (All volumes are measured at STP)
Solution:
Step 1: Write a balanced equation for the reaction.S (s) + O2 (g) → SO2 (g)
Step 2: Get the number of moles from the grams.
32 g of sulfur atoms = 1 mole of sulfur atoms
So, 1 g = 1 ÷ 32 mole or 0.03125 moles of sulfur atoms
1 mole of sulfur atoms gives 1 mole of sulfur dioxide molecules
So, 0.03125 moles of sulfur atoms gives 0.03125 moles of sulfur dioxide
Step 3: Get the volume.
1 mole of sulfur dioxide molecules has a volume of 22.4 at STP
So, 0,03125 moles has a volume of 0.03125 × 22.4 = 0.7 liters at STP
So, 0.7 liters of sulfur dioxide are produced.
How to solve equation stoichiometry questions with gases?
Examples and practice problems of solving equation stoichiometry questions with gases. We calculate moles with 22.4 L at STP, and use molar mass (molecular weight) and mole ratios to figure out how many products or reactants we have.
Example:
How many grams of H2O will be produced by 58.2L of CH
 4 at STP? Assume an excess of O2. Examples and practice problems of solving equation stoichiometry questions with gases. We calculate moles with the Ideal Gas Law, because the conditions are not at STP, and use molar mass (molecular weight) and mole ratios to figure out how many products or reactants we have.
4 at STP? Assume an excess of O2. Examples and practice problems of solving equation stoichiometry questions with gases. We calculate moles with the Ideal Gas Law, because the conditions are not at STP, and use molar mass (molecular weight) and mole ratios to figure out how many products or reactants we have.Example:
If 85.0 g of NaN3 decomposes at 75°C and 2.30 atm, what volume of N2 will be made?
- Show Step-by-step Solutions
Try the free Mathway calculator and problem solver below to practice various math topics. Try the given examples, or type in your own problem and check your answer with the step-by-step explanations.
We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
05th Apr 2019 @ 11 min read
Avogadro's law is also known as Avogadro's hypothesis or Avogadro's principle. The law dictates the relationship between the volume of a gas to the number of molecules the gas possesses. This law like Boyle's law, Charles's law, and Gay-Lussac's law is a specific case of the ideal gas law. This law is named after Italian scientist Amedeo Avogadro. He formulated this relationship in 1811. After conducting the experiments, Avogadro hypothesized that the equal volumes of gas contain the equal number of particles. The hypothesis also reconciled Dalton atomic theory. In 1814 French Physicist Andre-Marie Ampere published similar results. Hence, the law is also known as Avogadro-Ampere hypothesis.
Statement
For an ideal gas, equal volumes of the gas contain the equal number of molecules (or moles) at a constant temperature and pressure.
In other words, for an ideal gas, the volume is directly proportional to its amount (moles) at a constant temperature and pressure.
Explanation
As the law states: volume and the amount of gas (moles) are directly proportional to each other at constant volume and pressure. The statement can mathematically express as:
Replacing the proportionality,
where k is a constant of proportionality.
The above expression can be rearranged as:
The above expression is valid for constant pressure and temperature. From Avogadro's law, with an increase in the volume of a gas, the number of moles of the gas also increases and as the volume decreases, the number of moles also decreases.
If V1, V2 and n1, n2 are the volumes and moles of a gas at condition 1 and condition 2 at constant temperature and pressure, then using Avogadro's law we can formulate the equation below.
Let the volume V2 at condition 2 be twice the volume V1 at condition 1.
Therefore, with doubling the volume, the number of moles also gets double.
The formation of water from hydrogen and oxygen is as follows:
$underset{1,text{mol}}{ce{H2O}}$}' alt='Water reaction'>In the above reaction, 1 mol, (nH2) of hydrogen gas reacts with a 1⁄2 mol (nO2) of oxygen gas to form 1 mol (nH2O) of water vapour. The consumption of hydrogen is twice the consumption of oxygen which is expressed below as:
Let say, 1 mol of hydrogen occupies volume VH2, a 1⁄2 mol of oxygen occupies VO2 and similarly for 1 mol of water vapour, volume VH2O. As we know from Avogadro's law, equal volumes contain equal moles. Hence, the relationship between the volumes is the same as among the moles as follows:
Avogadro's law along with Boyles' law, Charles's law and Gay-Lussac's forms ideal gas law.
Graphical Representation
The graphical representation of Avogadro's law is shown below.
The above graph is plotted at constant temperature and pressure. As we can observe from the graph that the volume and mole have a linear relationship with the line of a positive slope passing through the origin.
As shown in the above figure, the line is parallel to the x-axis. It means that the value of volume by mole is constant and is not influenced by any change in mole (or volume).
Both the above graphs are plotted at a constant temperature and pressure.
Avogadro's constant
The Avogadro's constant is a constant named after Avogadro, but Avogadro did not discover it. The Avogadro's constant is a very useful number; the number defines the number of particles constitutes in any material. It is denoted by NA and has dimension mol−1. Its approximate value is given below.
Molar Volume
Since Avogadro's law deals with the volume and moles of a gas, it is necessary to discuss the concept of molar volume. The molar volume as from the name itself is defined as volume per mole. It is denoted as Vm and having a unit of volume divided by a unit of mole (e.g. dm3 mol−1, m3 kmol−1, cm3 mol−1 etc). From the ideal gas law, at STP (T = 273.15 K, P = 101 325 Pa) the molar volume is calculated as:
Limitation of Avogadro's law
The limitation are as follows:
- The law works perfectly only for ideal gases.
- The law is approximate for real gases at low pressure and/or high temperature.
- At low temperature and/or high pressure, the ratio of volume to mole is slightly more for real gases compare to ideal gases. This is because of the expansion of real gases due to intermolecular repulsion forces at high pressure.
- Lighter gas molecules like hydrogen, helium etc., obey Avogadro's law better in comparison to heavy molecules.
Real World Applications of Avogadro's Law
Avogadro's principle is easily observed in everyday life. Below are some of the mentioned.
Balloons
When you blow up a balloon, you are literally forcing the air from your mouth to inside the balloon. In other words, you are filling more moles of air in the balloon and it expands.
Tyres
Have you ever filled deflated tyres? If yes, then you are nothing but following Avogadro's law. When you pump air inside the deflated tyres at a gas station, the amount (moles) of gas inside the tyres is increased which increases the volume and the tyres are inflated.
Human lungs
When we inhale, air flows inside our lungs and they expand while when we exhale, the air flow from the lungs to surroundings and the lungs shrink.
Laboratory Experiment to prove Avogadro's law
Objective
To verify Avogadro's law by estimating the amount (moles) of different gases at a fixed volume, temperature and pressure.
Apparatus
The apparatus requires for this experiment is shown in the above diagram. It consists of a U-tube manometer (in the diagram closed-end manometer is used, but opened-end manometer can also be used) as depicted in the figure, mercury, a bulb, a vacuum pump, four to five cylinders of different gases and a thermometer. Connect the all apparatuses as shown in the figure.
Nomenclature
- V0 is the volume of the bulb, which is known (or determined) before the experiment.
- T is the temperature at which the experiment is performed, which can be determined from the thermometer (for simplicity take it as room temperature).
- P is the pressure at which the experiment is performed, which can be determined from the difference in heights of mercury level in the manometer.
- W0 is the empty weight of the bulb, and it is known (or determined) before the experiment.
- W is the filled weight of the bulb.
- Wg is the weight of the gas inside the bulb.
- M is the molar mass of the gas.
Procedures

- Take a gas cylinder attached it the bulb setup and also attached the pump to the bulb setup. Care must be taken while attaching the apparatus to prevent any leakages of the gas.
- First, close the knob of the gas cylinder and open the vacuum pump knob on the bulb. Evacuate the air filled in the system and by turning on the vacuum pump.
- Once the bulb is emptied, close the vacuum pump knob and switch off the vacuum pump.
- Start filling the bulb with the cylinder gas by opening the gas cylinder knob slowly until the desired difference in the mercury height is achieved. Note the height difference in the manometer. (The value of the height difference should be the same for all the readings.)
- Close all the knobs, also close the connection between the bulb and the manometer to isolate the gas inside the bulb. Disassemble the bulb from the manometer.
- Weigh the bulb on a weighing machine and note the reading down.
- This finishes the procedure for the first gas. Repeat the same procedure for different gases.
Calculation
Calculate the weight of gas (Wg) in the bulb by subtracting the weight of empty bulb (W0) from the weight of the filled bulb (W).
Then calculate the number of moles of the gas as:
The number of moles of all gases should be approximately equal within a small percentage of error. If this is true, then all the gases do obey the Avogadro's law.
If the experiment is performed at STP (T = 273.15 K, P = 101 325 Pa) , then we can also calculate the molar volume Vm as:
And its value should be close to 22.4 dm3 mol−1.
Examples
Example 1
Consider 20 mol of hydrogen gas at temperature 0 °C and pressure 1 atm having the volume of 44.8 dm3. Calculate the volume of 50 mol of nitrogen gas, at the same temperature and pressure?
As from Avogadro's law at constant temperature and pressure,
Therefore, the volume is 112 dm3.
Example 2
There is the addition of 2.5 L of helium gas in 5.0 L of helium balloon; the balloon expands such that pressure and temperature remain constant. Estimate the final moles of gas if the gas initially possesses 8.0 mol.
The final volume is the addition of the initial volume and the volume added.
From Avogadro's law,
The final number of moles in 7.5 L of the gas is 12 mol.
Example 3
3.0 L of hydrogen reacts with oxygen to produce water vapour. Calculate the volume of oxygen consumed during the reaction (assume Avogadro's law holds)?
For the consumption of every one mole of hydrogen gas, half a mole of oxygen is consumed.
As per Avogadro's law, the volume is directly proportional to moles, so we can rewrite the above equation as:
1.5 L of oxygen is consumed during the reaction.
Associated Articles
 If you appreciate our work, consider supporting us on ❤️ patreon
If you appreciate our work, consider supporting us on ❤️ patreonAvogadro's Gas Law Problems
 .
.Using Avogadro's Law
- 9
- cite
- response
Copy Article Cite
What Is Avogadro's Law
